BiXAb® TECHNOLOGY
BIOMUNEX has created a best in class, robust bispecific antibody technology platform, BiXAb®, thanks to a proprietary computational modelling approach. Being based on an IgG format, BiXAb® antibodies are endowed with natural drug-like properties and have excellent biophysical and manufacturing properties.
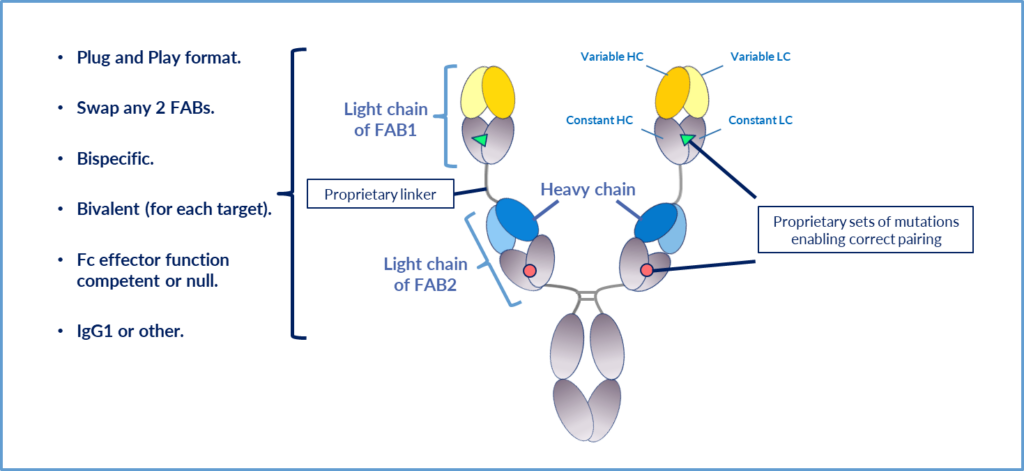
BIOMUNEX’ BiXAb® technology platform has been designed to incorporate the Fabs from any pair of monoclonal antibodies in a simple “Plug and Play” format. This permits the rapid generation of BiXAb antibodies, with no additional engineering, in less than 6 weeks, affording unsurpassed speed for BIOMUNEX and partners to create new compositions of matter and protect them in a time efficient manner.
BiXAb Features
Speed
The modular “Plug and Play” system employed for the construction of BiXAb antibodies leads to extremely short generation times. From Fab sequence identification made in silico to BiXAb purification and characterization is typically less than 6 weeks.
Excellent Pairing
To ensure correct pairing of the two Light chains, a series of proprietary mutations (pairs) have been designed (described and covered by two patent families), in the constant regions of the Fabs that can be used in various combinations to insure correct light chain:heavy chain pairing.
Linkers
A series of proprietary linkers, based on natural human immunoglobulin sequences, have been designed (described and covered by two patent families), that have sufficient length and flexible rigidity to permit efficient binding of the internal Fab (verified experimentally in many experiments; see article published in Frontiers in Immunology by Rabia et al 2023). These linkers also contribute significantly to the many advantageous biophysical and functional properties of these bi-specific antibodies.
Valency
The BiXAb technology has been initially developed as a bivalent (per target) and bi-specific antibody platform possessing tetra-valency overall. This permits the BiXAb antibodies to bind targets with affinities similar to native monoclonal antibodies and profits from the advantages of “avidity driven binding” seen with normal Mabs. This can be particularly important when one of the targets has poor expression.
Excellent Drug-like Properties
The excellent drug-like properties of the BiXAb antibodies are due to the fact that the format is based on a native IgG structure with the internal Fab linked to a second correctly assembled Fab attached by a natural, immunoglobulin-derived linker sequence. These properties include excellent binding affinities, lack of aggregation, low risk of immunogenicity and high thermal stability.
Robust Manufacturability
Due to the robust biochemical and biophysical features of the “naturally folded” BiXAb structure, excellent manufacturability in CHO cells is achieved with the BiXAb format. This can have a major impact on CMC efficiency, cost of goods and clinical supply.
BIOMUNEX has developed a very strong IP protection position and is continuously solidifying patent protection of its BiXAb technology as well as the innovations and improvements that continue to differentiate the BiXAb technology from most of those used in the bsAb field. The BiXAb® technology changes the paradigm of the multi-specific antibody field: due to its rapid design and efficiency, the BiXAb can be used to identify the most synergistic pairs of targets for a new multi-specific antibody and rapidly generate new IP created from mAbs in a cost-effective manner.